US FDA have start accepting PADER submission in eCTD format from June 10, 2015. Sarjen provides predefined template for PADER submission so that fast submission of adverse event can be reported to US FDA.
US FDA issued a final rule on June 10, 2014, that requires industry to submit post marketing safety reports in an electronic format. The final rule and accompanying draft guidance, providing submissions in electronic format—post marketing safety reports, applies to virtually all post marketing safety reports for human drug and biologic products, which includes individual case safety reports (ICSRs) and periodic safety reports.
The final rule for PADER Submission issued with a draft guidance for industry. US FDA will start accepting PADER submission in eCTD format from June 10, 2015.
Periodic Adverse Drug Experience Reports (PADERs) in the U.S. are submitted to update and evaluate the worldwide safety experience with a medicine at defined time points after approval. These reports provide summary information together with an evaluation of the benefit-risk profile of approved medicines in the light of new or changing post-approval information.
This evaluation is designed to help ascertain whether further investigations are necessary and whether changes should be made to the approval or to the medicine’s labeling.
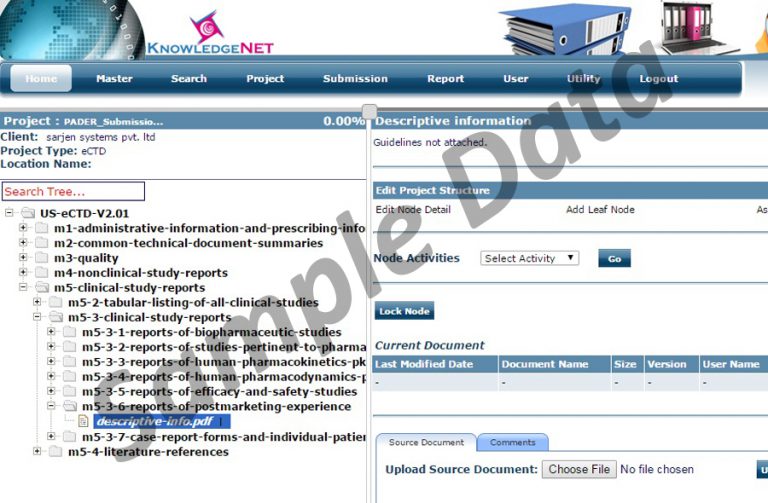
PADERs are submitted to the individual eCTD-based applications in PDF format. The PADER is provided as a single pdf file with proper bookmarks, table of contents and hyperlinks in the eCTD section, m5.3.6.