Draft Policy on Designating Regulators as Listed Authorities (WHO-Listed Authority) by WHO
The World Health Organization’s vision towards strengthening the regulation of medical products in underserved regions, the WHO published a draft policy on Designating Regulators as Listed Authorities (WHO-Listed Authority) to establish a framework for ensuring certain medical product regulators can be globally recognized as meeting WHO and other international standards and practices.
World Health Assembly Resolution on Regulatory system strengthening for medical products recognizes that effective regulatory systems are an essential component of health system strengthening, contribute to better public health outcomes and are necessary to the implementation of universal health coverage. The Resolution also recognizes that inefficient regulatory systems can be a barrier to access to safe, effective and quality medical products.
The introduction of a framework for designating and publicly listing a regulatory authority as a WHO-Listed Authority (WLA) provides a transparent and evidence-based pathway for regulatory authorities to be globally recognized as meeting WHO and other international recognized standards and practices, replacing the concept of a stringent regulatory authority (SRA) which was initially developed to guide global procurement of medicines. Under the framework, if a regulator passes a WHO-Listed Authority (WLA) evaluation process, the regulator will be publicly listed alongside the scope of the designation (product types and/or regulatory functions); evidence reviewed, and process undertaken to support the listing; and the period of validity of the listing. A listing will normally be valid for a period of at least five years, the draft says, provided there have not been any changes that could negatively impact the regulator’s listing.
The principle of reliance is central to WHO’s approach to regulatory system strengthening and effective regulation, regardless of the size and maturity of the authority. The proposed framework uses an evaluation tool (known as Global Benchmarking Tool or GBT) to generate and analyze the evidence of a regulatory system’s performance. WHO is also developing a system for evaluating the performance of regional regulatory networks or systems.
Only regulators with a maturity level 3 authority will be eligible for consideration as a WHO-Listed Authority (WLA).
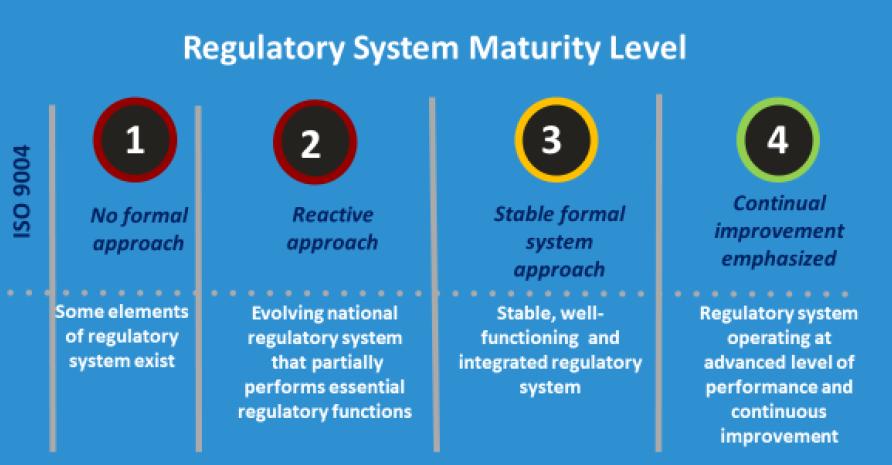
WHO also clarified that the designation of WHO-Listed Authorities (WLAs) is meant to substantiate their maturity and performance as defined by the GBT and the performance evaluation process. But the framework is not meant to make any inference regarding the maturity or performance of a regulatory authority that has not been evaluated by WHO.
For more detail please refer below link:
